Combustion and Flame
- Gratitude miracles

- Dec 12, 2020
- 8 min read
Updated: Dec 16, 2020
Class 8
Chapter 6
Combustion: A chemical process in which a substance reacts with oxygen (of air) to give heat and light.
The light which is given off during combustion can be in the form of a flame or as a glow.
For example: *wood burns by producing flame
*During respiration, glucose burns in the presence of oxygen producing heat energy and waste products like carbon dioxide and water.
C6H12O6 + O2 -----> CO2 + H2O + Energy
Combustible substance: A substance that burns in the presence of oxygen(of air) to produce heat and light.
For example: paper, wood, kerosene, LPG, CNG, coal, petrol, diesel etc.
Non combustible substance: A substance that does not burn in the presence of oxygen(of air) is called non combustible substance.
Example: stone, bricks, cement, sand, soil, copper objects etc.
*Combustion is a chemical reaction because during the combustion the substance burns in presence of oxygen to form heat and light.
Charcoal burns in air to give carbon dioxide and heat.
C + O2 → CO2 + Heat
Products of combustion
Fuels are mostly made up of hydrocarbons. When hydrocarbon burns completely it forms carbon dioxide and water. Heat and light energy are released.
CH4 + 2O2 ---> CO2 + 2H2O + energy (sufficient supply of oxygen).
When incomplete combustion(sufficient oxygen is not available) of fuel takes place
, it form carbon monoxide, water and energy.
2CH4 + 3O2 ---> 2CO + 4H2O + energy (insufficient supply of oxygen).
That's why it is not advisable to burn coal in closed room and sleep at night.
Conditions necessary for combustion
* Combustible substance
*Oxygen (a supporter of combustion):
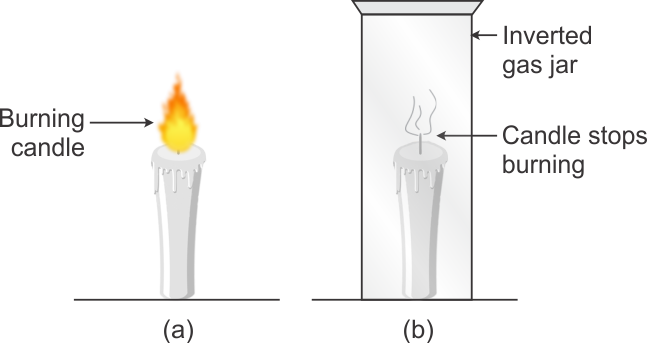
Activity : Air is needed for combustion.
Activity: Take a candle and fix it on a table. The candle is lighted. The candle will continue to burn due to continuously available fresh air providing the required oxygen for combustion.
Now cover the burning candle by putting an inverted gas jar over it. After a short time, the candle stops burning and gets extinguished. When the burning candle is covered with gas jar, then the candle takes away the oxygen necessary for burning from the air enclosed in the gas jar. After some time, when all the oxygen of air inside the gas jar is used up, then the burning candle gets extinguished. This proves that air is necessary for combustion of substances.
Q Does a matchstick burn by itself? How does it burn?
Q You must have had an experience of burning a piece of paper. Does it burn when a burning matchstick is brought near it?
Q Can you burn a piece of wood by bringing a lighted matchstick near it? Why do you have to use paper or kerosene oil to start fire in wood or coal? Have you heard of forest fires?
* Ignition temperature: The lowest temperature at which a substance catches fire and starts burning. Different substance have different ignition temperature. That's why they burn at different temperature.
Activity 3: Combustion and Ignition temperature.
Make two paper cups by folding a sheet of paper. Pour about 50 mL of water in one of the cups. Heat both the cups separately with a candle . What do you observe?

The heat supplied to the paper cup is transferred to water by conduction. So, in the presence of water, the ignition temperature of paper is not reached. Hence, it does not burn.
* During extreme heat of summer, at some places dry grasses catch fire. From grasses, it spreads to trees, and very soon the whole forest is on fire (Fig. 6.4). It is very difficult to control such fires.
a) Some of the substances having low ignition temperature like
Paper- 450 °C
Alcohol- 365 °C
Petrol- 280 °C
Inflammable substances: The substances which have very low ignition temperature and can easily catch fire with a flame. For example: Petrol, alcohol, LPG, CNG.
Types of combustion:
Rapid combustion: The combustion reaction in which a large amount of heat and light are produced in a short time is called rapid combustion. Substances having low ignition temperature undergoes rapid combustion.
For example: Burning of cooking gas (LPG) in a gas stove to give heat and light.
Burning of Kerosene oil in the kerosene stove and burning of wax in a candle.
Spontaneous combustion: The combustion reaction which occurs on its own without the help of any external heat is called spontaneous combustion. It takes place at the room temperature.
For example: White Phosphorus, sodium and potassium is a substance which undergoes a spontaneous combustion.
Explosive combustion: A very fast combustion reaction in which a large amount of heat light and sound are produced. A large amount of gases released quickly in an explosive combustion. It is the rapid expansion of these gases which cause a loud sound explosion.
For example: Burning of crackers on diwali.
EXTINGUISHING A FIRE
The three conditions necessary for a fire are:
Presence of combustible substances
Presence of oxygen
Heating the combustible substance above its ignition temperature.
The methods of extinguishing a fire involves the following:
Removing the combustible substance: Cylinder, wooden stuff, papers, kerosene, oil etc.
Cooling the substance to below its ignition temperature: It is done with the help of water, but water works only on wood or paper are on fire.
Exception: Fire in electrical equipments cannot be controlled with water as water conducts electricity due to the presence of salts in it and person can get an electric shock.
Exception: Oil or petrol fire cannot be put out by water as oil or petrol are lighter than water and they float above the water and keep burning.
3. Cutting of the supply of air: This can be done with the help of carbon dioxide gas, which is not the supporter of combustion. Besides, it is heavier than air and forms a blanket around the fire. This method works well for electrical and petrol fires.
Q How do we get the supply of carbon dioxide from fire extinguisher?
Ans. It can be stored at high pressure as a liquid in cylinders. In what form is the LPG stored in cylinders? When released from the cylinder, CO2 expands enormously in volume and cools down. So, it not only forms a blanket around the fire, it also brings down the temperature of the fuel. That is why it is an excellent fire extinguisher.
Another way to get CO2 is to release a lot of dry powder of chemicals like sodium bicarbonate (baking soda) or potassium bicarbonate. Near the fire, these chemicals give off CO2
Soda - acid type fire extinguisher: In this , the property of acids to liberate carbon dioxide on reacting with carbonates and bicarbonates of metals is utilized.
2NaHCO3 + H2SO4 ------> Na2SO4 + 2H2O + 2CO2
Q Difference between rapid and spontaneous combustion
Q Forest fires are a result of which type of combustion and why ?
FLAME
FLAME: A flame is a region where combustion of gaseous substances takes place.
Q Why do some substances burn with a flame and some do not?
Ans. 1. The combustible substances like wax, which vaporize on burning produce flames. And those combustible substances which do not get vaporize on burning do not produce flame. All the gases which undergo combustion produce a flame, but only solid and liquid fuels which vaporise on heating burns with a flame.
For example: Kerosene, wax, wood burns with flame.
Coal and coke do not produce flame while burning.

Structure of a flame In order to understand the structure of a flame, light a wax candle and watch its flame. Carefully note the different coloured zones in the flame. Starting from the base of the flame, a flame has three zones.

* The outermost zone is the hottest among all zones and is blue in colour, and this is due to complete combustion. It is the non-luminous part of the flame.
The middle zone of the candle flame is moderately hot and is yellow in colour, and partial combustion of fuel takes place. It is the bright part of the flame.
The innermost zone of the flame is the least hot and is black in colour. This is due to the presence of unburnt wax vapours.
Test Yourself 1. Which one of the following is fuel of our body? (a) Petrol (b) Diesel (c) Food (d) Water 2. Name the currency of energy in our body. Booster 5 Why do goldsmiths use the outermost zone of the flame for melting gold and silver? Explanation: The goldsmith uses the outermost zone of the flame to use for melting gold and silver because it is the hottest part of the flame and undergoes complete combustion. Q What is Fuel? Any substance which is easily available and burns in air at a moderate rate, producing a large amount of heat energy, without leaving behind any undesirable residue is called fuel. Example: wood, charcoal, petrol, kerosene, etc. Booster 6 Why is sulphur not used as a fuel even though it can burn in air to produce heat? Explanation Sulphur is easily available in nature and can burn in air to produce heat. However, it is not a fuel because on burning, it forms a poisonous gas, SO2 , which can cause serious respiratory problems and can even be fatal. Ideal Fuel There is probably no fuel that could be considered as an ideal fuel. We should look for a fuel which fulfils most of the requirements for a particular use. Characteristics of a good fuel (1) It should be cheap and readily available. (2) It should be easy to store. (3) It should burn at a slow rate and its combustion should be controllable (4) It should have low ignition temperature. (5) It should produce very small amount of residues such as ash. (6) It should have large calorific value. (7) It should not produce gases which pollute the air. (8) It should not produce any hazards during transportation. Classification of fuels On the basis of physical state, fuels are classified into three parts. 1. Solid fuels: The fuels which occur In a solid state at room temperature are called solid fuels. Example: Wood, agricultural residues, charcoal, coal, coke, etc. 2. Liquids fuels: The fuels which occur in a liquid state at room temperature are called liquid fuels. Example: Liquefied hydrogen, petrol, oil, kerosene, diesel, etc. 3. Gaseous fuels: The fuels which occur in a gaseous state at room temperature are called gaseous fuels. Example: Water gas, producer gas, coal gas, compressed natural gas (CNG) and gobar gas, etc. Fuel efficiency The amount of heat energy produced on completely burning one Kilogram of fuel is called the calorific value of a fuel. The more is the calorific value of a fuel, more is the efficiency of the fuel. The calorific value of the fuels is expressed in Kilojoules per kilogram (kJ/kg) or kilojoules per gram (kJ/g).
Calorific Values of Different Fuels Fuel
Calorific Value (kJ/kg)
Cow dung cake 6000-8000
Wood 17000-22000
Coal 25000-33000
Petrol 45000
Kerosene 45000
Diesel 45000
Methane 50000
CNG 50000
LPG 55000
Biogas 35000-40000
Hydrogen 150000
Harmful effects of burning fuels The increasing fuel consumption has harmful effects on the environment. The main products formed during the fuel combustion which produce harmful effect are: 1. Carbon fuels like wood, coal, petroleum release unburnt carbon particles. These fine particles are dangerous pollutants causing respiratory disease, such as asthma. 2. Incomplete combustion forms carbon monoxide gas. It is very poisonous gas. It is dangerous to burn coal in a closed room. The carbon monoxide gas produced can kill persons sleeping in that room. 3. Combustion of most fuels releases carbon dioxide in the environment. Increased percentage of carbon dioxide in the air causes global warming. Global warming is the rise in temperature of the earth. This result in melting of polar glaciers. This leads to rise in sea level and floods in the sea coast. 4. Burning of coal and diesel release sulphur dioxide gas. It is an extremely suffocating and corrosive gas. Sulphur dioxide and nitrogen oxide dissolve in rain water to form acid. Such rain is called acid rain. It is very harmful for crops, buildings and soil. 5. Wood is also used as a fuel. Burning of wood gives a lot of smoke which causes air pollution and is also very harmful for humans. It may lead to many respiratory problems. Cutting of trees for obtaining wood Leads to deforestation which is quite harmful to environment. 6. Carbon particles of smoke or the ash get suspended in the air. Excessive amount of them in the air causes breathing problems.
How to prevent pollution
The use of diesel and petrol as fuels in automobiles is being replaced by CNG (Compressed Natural Gas), because CNG produces the harmful products in very small amounts. CNG is a cleaner fuel.
ACTIVITY
Make a model of a fire extinguisher. Place a short candle and a slightly taller candle in a small dish filled with baking soda. Place the dish at the bottom of a large bowl. Light both the candles. Then pour vinegar into the dish of baking soda. Take care. Do not pour vinegar on the candles.



Comments