Class 10- Periodic classification of elements
- Gratitude miracles

- Nov 11, 2020
- 9 min read
Updated: Jan 16, 2021
Chapter 5 - Periodic classification of elements
It is very difficult to study the property of 118 elements separately so the element with the similar properties are classified into the same group. In this way study of a large number of element is reduced to the study of few groups of the elements
Dobereiner's Triads
Dobereiner's was the first scientist to show the relationship between the properties of elements and their atomic masses.
According to the Dobereiner's law of Triads:
*When elements are arranged in order of increasing atomic masses, groups of three elements having the similar chemical properties are obtained.
* The atomic mass of the middle element of the triad being equal to the arithmetic mean of the atomic masses of other two elements.
Group 1.
*The alkali metal group
Element Atomic mass
Lithium 7
Sodium 23
Potassium 39
Atomic mass of the sodium is equal to to the average atomic mass of Lithium and potassium - 7+39/2 = 23.
*All the elements are metal
*They react with water to form the alkaline(basic) solution liberating hydrogen gas.
2Na + 2H2O ------> 2NaOH + H2
* All have valency 1 because all of them have a tendency to lose one electron to complete their octet.
Na - K L M
11- 2 8 1
Li - 2 1
Group 2
*The alkaline earth metal group
Element Atomic mass
Calcium 40
Strontium 88
Barium 137
Atomic mass of the strontium is equal to to the average atomic mass of calcium and barium - 40+137/2 = 88.5
*All the elements are metals
*The oxides of all the elements are alkaline(basic) in nature.
* All the element have the valency of 2 because all of them have a tendency to lose 2 electrons to complete their octet.
Ca - K L M N
20 - 2 8 8 2
Group 3
The halogen group
Element Atomic mass
Chlorine 35.5
Bromine 80
Iodine 127
Atomic mass of the bromine is equal to to the average atomic mass of chlorine and iodine - 35.5+127/2 = 81.2
*All the elements are nonmetals
*The halides(compounds with hydrogen) of all the elements are acidic HCl, HBr, HF.
*All the elements have the valency of -1 because they have tendency to gain one electron to complete their octet.
Cl - K L M
17 - 2 8 7
Limitations of Dobereiner's Triads:
All the known elements are not arranged in the triads.
Even in the same the law failed. For example:
Halogens: F Cl Br
Atomic mass: 19 35.5 80
Atomic mass of the chlorine should be equal to to the average atomic mass of fluorine and bromine - 19+80/2 = 45.5
3. Although nitrogen, phosphorus and arsenic exhibit similar chemical properties, they do not constitute a Dobereiner's triads.
Element Atomic mass
Nitrogen 14
Phosphorus 31
Arsenic 74.9
Atomic mass of the phosphorus is not equal to to the average atomic mass of nitrogen and arsenic - 14+74.9/2 = 44.45
Hence nitrogen, phosphorus and arsenic do not constitute a Dobereiner's triads in spite of similar properties.
Assignment based on Dobereiner's Triads:
Q1. Which of the following will not represent the Dobereiner's Triads ?
a) Li, Na, K b) Be, Mg, Ca c) Cl, Br, I d) N, P, As
Q2. What was the limitation of Dobereiner law of triads?
Q3. Why do Li, Na and K how resemblance on the basis of Dobereiner’s law of triads?
Q4. A, B and C are elements of Dobereiner’s triad. If the atomic mass of A is 7 and that of C is 23, what will be the atomic mass of B.
Q5. Classify the following elements into groups of triads
Cl, Ca, Ba, Sr, K, Li, Br, I, Na
Q6. Can the following groups of elements be classified as a :
1) Na, Si, Cl 2) Be, Mg, Ca
Atomic mass Be- 9, Na - 23, Mg - 24, Si - 28, Cl - 35, Ca - 40
Justify your answer in each case. [CBSE 2019]
Newlands’ Law of Octaves:
According to this ‘when elements are placed in order of increasing atomic masses, the physical and chemical properties of every 8th element are a repetition of the properties of the first element starting from anywhere.
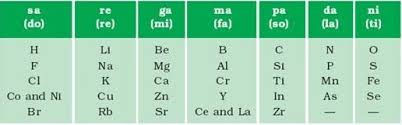
Merits:
1. First time the elements are arranged in increasing atomic weight {relationship between atomic mass and properties of an element}.
2. Lighter elements were grouped together till atomic mass 40 (Calcium).
Limitations
Law of octaves was applicable only upto calcium (only for lighter elements).
Newland adjusted two elements in the same slot (e.g. Co and Ni), having different properties. For example; Co and Ni with Fluorine, Chlorine, Bromine and Iodine.
Newland placed Fe away from Co and Ni, although Fe resembles Co and Ni in properties.
According to Newland, only 56 elements existed in nature and no more elements would be discovered in future.
No space was left for the noble gas elements.
Mendeleev’s Periodic Law:
According to this “The physical and chemical properties of the elements are the periodic function of their atomic masses.”
Periodicity of Properties: The repetition of properties of elements after certain regular intervals is known as Periodicity of Properties.
Merits of Mendeleev’s Periodic Table
* There were 6 periods(horizontal rows) and 8 groups(vertical column) in the original table of Mendeleev's. The groups were numbered I to VIII. The group I to VII were divided into subgroup A and B and group VIII housed triads of elements without any division into subgroup A and B.
* Mendeleev’s left vacant places in his table which provided an idea for the discovery of new elements. Example: Eka-boron(Scandium - Sc), Eka-aluminium(Gallium - Ga) and Eka-silicon(Germanium- Ge).
Properties of eka - aluminium and gallium
Property eka-aluminium gallium
Atomic mass 68 69.7
Formula of oxide E2O3 Ga2O3
Formula of chloride ECl3 GaCl3
* Mendeleev’s periodic table was predicted properties of several undiscovered elements on the basis of their position in Mendeleev’s periodic table.
* Noble gases could accommodate in the Mendeleev’s periodic table without disturbing the periodic table after discovery.

Limitations of Mendeleev’s Periodic Table (a) No fixed position for hydrogen: No correct position of the hydrogen atom was in Mendeleev’s periodic table.
Hydrogen has been placed in group I with alkali metals because like alkali metals , hydrogen also combines with halogens, oxygen and sulphur to form compounds having similar formulae and electronic configuration of both the elements resembles.
Hydrogen also resembles halogens , they both exists in the form of diatomic molecules.
hydrogen also combines with metals to form ionic compounds like NaH, KH Example: Position of hydrogen with alkali metals and halogens (17th group).
(b) No place for isotopes: Position of isotopes were not decided. Example: Cl-35 and Cl-37.
(c) No regular trend in atomic mass: Position of some elements with lower atomic masses before with higher atomic mass. Example: Ni-58.7 before Co-58.9.
The Modern Periodic Table:
In 1913, Henry Moseley showed that the atomic number of an element is a more fundamental property than its atomic mass.
Modern Period Law: The physical and chemical properties of elements are the periodic function of their atomic number. Modern periodic table is based on atomic number of elements. Atomic number (Z) is equal to the number of protons present in the nucleus of an atom of an element and this no. increases by one in going from one element to next.
Prediction of the properties of the elements could be made with more precision when elements are arranged on the basis of increasing atomic no.
Features of Modern Periodic Table
Modern periodic table contains 18 vertical column known as group and seven horizontal rows known as periods.

Period 1 - Shortest period - 2 Elements- last electron enters in first shell( Accommodate max. 2 Electrons)
Period 2 - Short Period - 8 Elements - Last electron enters in L shell (Accommodate max. 8 electrons)
Period 3 - Short Period - 8 Elements - Last electron enters in M shell (Accommodate max. 18 electrons so theoretically it has 18 elements since the outermost shell cannot hold more than 8 electrons, so it has only 8 elements).
Period 4 - Long Period - 18 Elements
Period 5 - Long Period - 18 Elements
Period 6 - Long Period - 32 Elements
Period 7 - Long Period - 32 Elements (Incomplete period)
Representative Elements: The elements of groups 1,2 and 13 to 18 are called representative elements.
The chemical properties of the representative elements are determined by the no. of valence electrons in their atoms.
Noble Gases: Elements of group 18.
Transition Series: The elements of groups 3 to 12 are called transition elements.
Inner transition Elements: The elements placed at the bottom of the periodic table.
Features of Periods
1. On moving from left to right in a period, the number of valence electrons increases from 1 to 8 in these elements, but they contain the same no. of shells.
No. of valence shell electrons increases by one unit, as the atomic no. increases by one unit on moving from left to right in a period.
Elements A.N. K L
Li 3 2 1
Be 4 2 2
B 5 2 3
C 6 2 4
N 7 2 5
O 8 2 6
F 9 2 7
Ne 10 2 8
On moving from top to bottom in a group, number of shell increases and all the elements contain same no. of valence electron.
Elements A.N. K L M N O
F 9 2 7
Cl 17 2 8 7
Br 35 2 8 18 7
I 53 2 8 18 18 7
2. Atoms of different elements with the same no. of occupied shells( K, L and M) are placed in the same periods.
For example:
Elements A.N. K L M
Na 11 2 8 1
Mg 12 2 8 2
Al 13 2 8 3
Si 14 2 8 4
P 15 2 8 5
S 16 2 8 6
Cl 17 2 8 7
Ar 18 2 8 8
Different periods have different no. of electrons which can be explained on the basis of filling the electrons into various shells.
K shell - 2n2 - 2 elements - First period
L shell - 2n2 - 8 elements - Second period
M shell - 2n2 - 18 but outermost shell can have only 8 electrons, so the third period has only 8 elements.
Trends in Modern Periodic Table:
Valency, Atomic size, metallic and non-metallic characters, and Electronegativity. (i) Valency: The valency of an element is determined by the number of valence electrons present in the outermost shell of its atom (i.e. the combining capacity of an element is known as its valency). In Period: On moving from left to right in a period, the valency first increases from 1 to 4 and then decreases to zero (0). Elements A.N. K L M Valency
Na 11 2 8 1 1
Mg 12 2 8 2 2
Al 13 2 8 3 3
Si 14 2 8 4 4
P 15 2 8 5 3
S 16 2 8 6 2
Cl 17 2 8 7 1
Ar 18 2 8 8 0
In Groups: On moving from top to bottom in a group, the valency remains same because the number of valence electrons remains the same.
Group 7: Halogens
Elements A.N. K L M N O Valency
F 9 2 7 1
Cl 17 2 8 7 1
Br 35 2 8 18 7 1
I 53 2 8 18 18 7 1
Example: Valency of first group elements = 1 Valency of second group elements = 2.
(ii) Atomic size: Atomic size refers to radius of an atom. It is a distance between the centre of the nucleus and the outermost shell of an isolated atom.

In Period : On moving from left to right in a period, atomic size decreases because nuclear charge increases. Due to the increase in the nuclear charge in the same shell which tends to pull the electrons closer to the nucleus and reduce the size of the atom.
Elements A.N. K L M
Na 11 2 8 1
Mg 12 2 8 2
Al 13 2 8 3
Si 14 2 8 4
P 15 2 8 5
S 16 2 8 6
Cl 17 2 8 7
Ar 18 2 8 8
Example: Size of second period elements: Li > Be > B > C > N > O > F
Point to know: The atomic size of noble gases in corresponding period is largest
due to presence of fully filled electronic configuration (i.e. complete octet).
In Group: Atomic size increases down the group because new shells are being added in spite of the increase in nuclear charge.
Elements A.N. K L M N O
F 9 2 7
Cl 17 2 8 7
Br 35 2 8 18 7
I 53 2 8 18 18 7 Example ; Atomic size of first group element : Li < Na < K < Rb < Cs < Fr Atomic size of 17th group elements : F < Cl < Br < I
(iii) Metallic character: It is the tendency of an atom to lose electrons.
In Period: Along the period from left to right, metallic characters decreases because a tendency to lose electron decreases due to the increase in nuclear charge.
Elements A.N. K L M
Na 11 2 8 1
Mg 12 2 8 2
Al 13 2 8 3
Si 14 2 8 4
P 15 2 8 5
S 16 2 8 6
Cl 17 2 8 7
Ar 18 2 8 8
Example: Metallic character of second period elements: Li > Be > B > C > N > O > F
In Group: Metallic character, when moving from top to bottom increases because the atomic size and tendency to lose electrons increases.
Elements A.N. K L M N O P
Li 3 2 1
Na 11 2 8 1
K 19 2 8 8 1
Rb 37 2 8 18 8 1
Cs 55 2 8 18 18 8 1
Example: First group element : Li < Na < K < Rb < Cs Non- metal are electronegative because they have tendency to form bond by gaining of electrons.
Electronegativity increases as we move from left to right in period.
Electronegativity decreases as we move from top to bottom in group.



Comments